Relational Wheel of the Elements
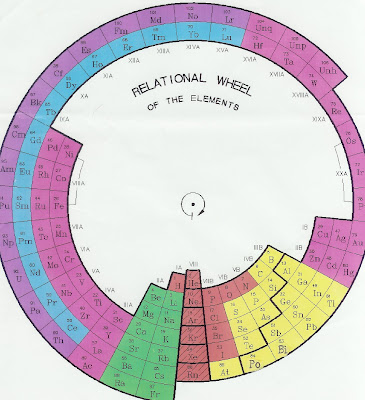
This is my new Relational Wheel of Elements which simply illustrates the proportional relationships between the different elements. Just click on the picture to blow it up bigger. You will notice that the Noble elements are in the vertical row starting with Helium. They have their electron shells completely filled with just the right number of electrons and that makes them compact and stable. Hydrogen is the smallest element by number of parts but its single loose electron floats around a lot and gives it a bigger atomic radius than Helium, so I put it a little farther away from the center of the wheel to denote its proportional size.
Notice that the elements on the left side of the vertical noble element row want to give up their extra electrons to fit into their standard electron shells and those elements on the right side of the noble elements want to absorb more electrons in order to fill out their standard electron shells. They would each like to be just like the stable noble elements and have just the correct number of electrons to pack inside their individual shells.
So if you look at the third ring out from the center of the wheel and you take the Sodium atom on the left of the stable Argon element and note that it has one too many electrons and put it near the Chlorine atom on the right side of the Argon element which needs one more electron, you can guess that by combining the two elements they will happily form the Sodium Chloride molecule (table salt) where the Sodium atoms loan their spare single electrons to the Chlorine atoms.
In a normal Periodic Chart of Elements there is no logical continuity. The list of elements go in a straight line from left to right by their increasing number of electrons until it gets to the noble element at the end of each row and then it just stops. It doesn't show the proportional jump in size to the next atom in the numerical series. It's like they fell off the edge of the Earth (joke).
I think that new Chemistry students will pick up on this relationship intuitively just by looking at the continuous loops in the Wheel of Elements and having it briefly explained to them. And I think that by teaching Chemistry with this wheel chart it will allow the students to pick up the concepts a day or two quicker than with the standard periodic chart. And later on if they use these principles in their work lives it should slightly improve their job performance by them picturing the relationships in their minds and correctly applying their knowledge.
The projected economic gain here is small at maybe an ongoing annual 0.0001% increase in GDP. But I think that by teaching science more logically it helps to make technology available to more people. We just want to make things easier and more profitable so we can get out into space quicker.
This Relational Wheel of Elements is original copyrighted content and is the intellectual property of the Space Vanguard SIG. You may use it for nonprofit educational purposes by disclosing its rightful owner.

Steve, unless my eyesight fails me, it appears that there is a small problem in that He is shown twice - once in its proper place, and once between Bi and Pb. It appears that in your chart, while Pb is correctly 82, Po which should be 84 is missing, with Bi in its place. He appears at 83.
ReplyDeleteCould you clarify this please?
DaSOB,
ReplyDeleteGood eye! Thanks, I'll get it corrected.
Steve